Orthotopic Brain Tumors in Mice
Orthotopic Brain Tumors in Mice
Orthotopic tumors models are created by directly injecting tumor cells or small tissue fragmentsinto the organ of interest in mice. For brain tumors, this involves implanting cancerous cellsdirectly into the brain parenchyma, allowing the tumor to grow within its natural environment(Figure 1). This is critical for studying brain cancers like glioblastomas, as the brain’s uniquearchitecture and immune environment cannot be accurately replicated in non-brain tissues.These models are also widely used in preclinical testing for therapeutic interventions, such aschemotherapy, radiation, and immunotherapy.
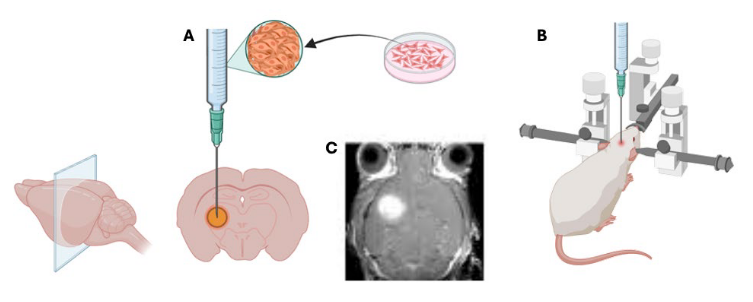
Figure 1. Schematic Representation of the Process of Generating an Orthotopic Model in the Brain of a Mouse, starting from cell culture to the localization of the injection site (A) using a stereotactic injection system, precisely targeting an exact location within the brain parenchyma (B). The effectiveness of the process can be monitored using methods such as Magnetic Resonance Imaging (C). These images were created using BioRender.com.
Brain orthotopic tumor models are particularly useful in investigating the molecular and cellulardynamics of tumor progression and metastasis in the brain. They allow us to examine howtumors invade surrounding brain tissues, interact with the immune system, and respond tohypoxia 1-3 . Several studies have shown the effectiveness of the Precision X-Ray, Inc. systems inOrthotopic Brain Tumors in MiceApplicationNoteFigure 1. Schematic Representation of the Process of Generating an Orthotopic Model in the Brain ofa Mouse, starting from cell culture to the localization of the injection site (A) using a stereotacticinjection system, precisely targeting an exact location within the brain parenchyma (B). Theeffectiveness of the process can be monitored using methods such as Magnetic Resonance Imaging (C).These images were created using BioRender.com. accurately targeting brain orthotopic tumors. For example, Salzillo et al. (2021) measured themetabolic evolution of glioblastoma during tumor development, regression, and recurrence intumor-bearing mice undergoing radiotherapy. On Days 25 and 27, the mice were both imagedand treated with 5 Gy whole-brain irradiation using the SmART+ (Small Animal Radio Therapy) Irradiator (Precision X-ray, Inc., Madison, CT) (Figure 2)4 .
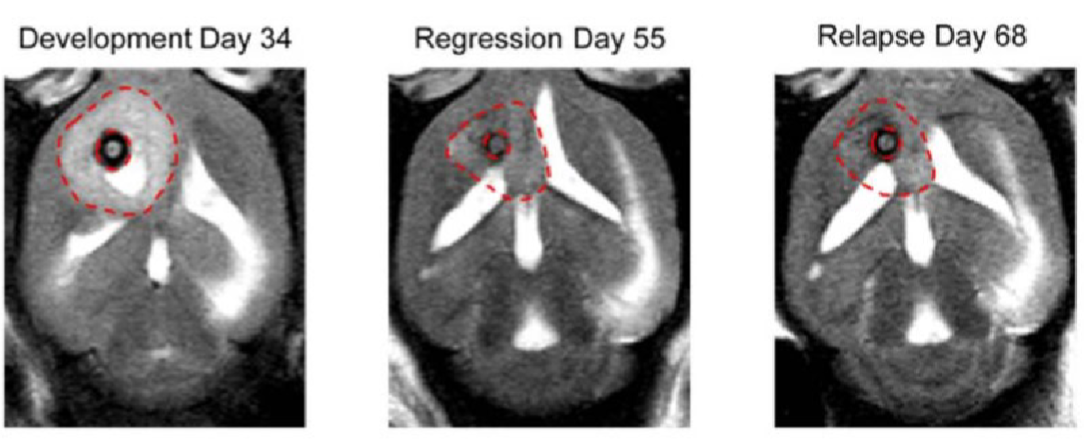
Figure 2. Anatomic and metabolic imaging of tumor-bearing mice over time. Tumor volume (A), imaged with T2-weighted MRI is displayed at the end of tumor development (Day 34), end of tumor regression (Day 55), and at the point of relapse (Day 68) in the same mouse. Adapted from (4).
Brain orthotopic models provide valuable insights into the mechanisms of radioresistance andthe potential for combination therapies, such as radiosensitizers or immune-modulating agents.Watson et al. (2024) analyzed the microenvironmental response to radiation therapy in apreclinical glioblastoma model, comparing it with a mouse model of breast-to-brain metastasis(Figure 3)5 . In this study, female C57BL6 mice were intracranially injected with a breast-to-brainmetastasis cell line at matched ages and cranial coordinates to initiate tumor growth. A single,targeted whole-brain dose of ionizing radiation (10-15 Gy) was administered using the Precision X-Ray SmART+ irradiator to assess the effects of radiation.
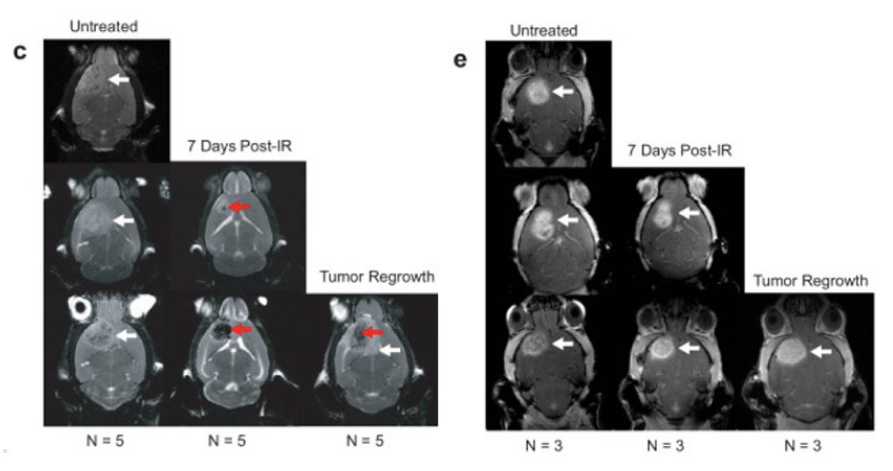
Figure 3. Single whole-brain focalized dose of ionizing radiation using the Precision X-Ray, Inc. SmART+ irradiator. Irradiation Treatment Sample Collection and Antibody Panel. Biweekly MRI monitoring tracked the process of regression and recurrence for each mouse following IR for (c) PDGfp (10 Gy ) and (e) BrM tumors (15 Gy ). White arrows indicate the tumor in both tumor types, red arrows indicate post-IR lesion in PDGfp tumors. Adapted from (5).
Conclusion
Orthotopic brain tumor models in mice are essential tools for advancing our understanding ofbrain cancer, as they closely replicate the unique environment in which brain tumors developand interact with surrounding tissues. These models are invaluable for assessing the efficacy ofdifferent therapeutic strategies, including X-radiation therapy, which is commonly used alone orin combination with chemotherapy or immunotherapy, in the clinical treatment of brain tumorslike glioblastoma.
References
1. Shi, W., Tanzhu, G., Chen, L., Ning, J., Wang, H., Xiao, G., … & Zhou, R. (2024). Radiotherapy inpreclinical models of brain metastases: a review and recommendations for futurestudies. International Journal of Biological Sciences, 20(2), 765..
2. Kloosterman, D. J., Erbani, J., Boon, M., Farber, M., Handgraaf, S. M., Ando-Kuri, M., … & Akkari,L. (2024). Macrophage-mediated myelin recycling fuels brain cancer malignancy. Cell, 187(19),5336-5356.
3. Yazdimamaghani, M., Kolupaev, O. V., Lim, C., Hwang, D., Laurie, S. J., Perou, C. M., … & Serody,J. S. (2025). Tumor microenvironment immunomodulation by nanoformulated TLR 7/8 agonist andPI3k delta inhibitor enhances therapeutic benefits of radiotherapy. Biomaterials, 312, 122750.
4. Salzillo, T. C., Mawoneke, V., Weygand, J., Shetty, A., Gumin, J., Zacharias, N. M., … &Bhattacharya, P. K. (2021). Measuring the metabolic evolution of glioblastoma throughout tumordevelopment, regression, and recurrence with hyperpolarized magnetic resonance. Cells, 10(10),2621.
5. Watson, S. S., Duc, B., Kang, Z., de Tonnac, A., Eling, N., Font, L., … & Joyce, J. A. (2024).Microenvironmental reorganization in brain tumors following radiotherapy and recurrencerevealed by hyperplexed immunofluorescence imaging. Nature Communications, 15(1), 3226.
