Why 160kVp Falls Short for Mice Irradiation
Why 160kVp Falls Short for Mice Irradiation Compared to Higher-Energy X-rays (225-350 kVp)
When evaluating total body irradiation (TBI) protocols for mice, the energy of the X-ray beamsignificantly influences the dose distribution, penetration depth, and uniformity of the radiationacross tissues, and is a critical factor in achieving effective, uniform, and reproducible results.
A comparison between 160 kVp and higher-energy X-rays (225-350 kVp) underscores why thelatter is more suitable for such studies. Key factors include penetration depth, dose uniformity,skin toxicity, and reliability. Here’s why 160 kVp X-rays fall short compared to higher-energy X-rays in the 225–350 kVp range:
Penetration Depth and Dose Distribution
- 160 kVp: Low-energy X-rays (like 160 kVp) have limited penetration depth, which is ofteninsufficient for uniform irradiation of the whole mouse body. This limitation can lead to higherdoses at the surface (skin and superficial tissues) and significantly lower doses in deeperorgans. As a result, some tissues might be under-irradiated, reducing the effectiveness of TBI.
- 225-350 kVp: Higher-energy X-rays penetrate tissues more effectively, allowing for a moreuniform dose distribution throughout the mouse’s body. This improved penetration depthhelps ensure that internal organs receive a consistent dose, which is critical for the reliabilityof experimental outcomes.
Skin Toxicity and Sparing Effect
- 160 kVp: Due to their lower energy, 160 kVp X-rays deposit a larger proportion of their doseat or near the surface, leading to higher skin dose relative to internal tissues. This can resultin significant skin toxicity, including erythema, dermatitis, or even skin necrosis, which canconfound results and reduce the viability of long-term studies.
- 225-350 kVp: Higher-energy X-rays provide a better skin-sparing effect, meaning the surfacedose is lower relative to the dose received by deeper tissues. This minimizes skin toxicity andallows a higher dose to reach internal organs, aligning better with the goals of TBI protocols.
Uniformity and Biological Response
- 160 kVp: With a less uniform dose distribution, biological effects can vary significantly acrossdifferent tissues. For instance, skin and bone may receive a substantially higher dose thaninternal organs. This variation can lead to inconsistent biological outcomes, complicatingstudies that require predictable whole-body radiation effects. This inconsistency cancomplicate systemic radiation studies, especially in radiation-sensitive mouse strains likeBALB/c.
- 225-350 kVp: A more uniform dose helps ensure that all tissues are irradiated similarly,providing more consistent biological responses. This uniformity is especially important forstudies examining systemic radiation effects, as it enables better control over dose-responserelationships.
Reproducibility and Reliability in Research
- 160 kVp: The inconsistent dose distribution from 160 kVp can hinder reproducibility, asbiological effects may not align consistently across experiments. Differences in energyabsorption between tissues could lead to variable outcomes that are less representative oftrue TBI.
- 225-350 kVp: The improved penetration and dose uniformity make these higher energiesmore reliable for reproducible results in TBI studies. This consistency is crucial forexperiments that aim to model human TBI, as higher-energy sources provide results that aremore comparable to clinical radiation protocols.
Preferred Protocols and Standards
- 160 kVp Limitations: While 160 kVp can be used for superficial or small-target irradiation, itis rarely recommended for TBI due to these limitations.
- 225-350 kVp as Standard: Many labs adopt those energies as the standard for mouse TBIbecause it balances sufficient penetration with dose uniformity, reducing complicationsrelated to skin toxicity or uneven tissue responses. This energy range is often seen as theoptimal choice for experimental consistency and biological accuracy.
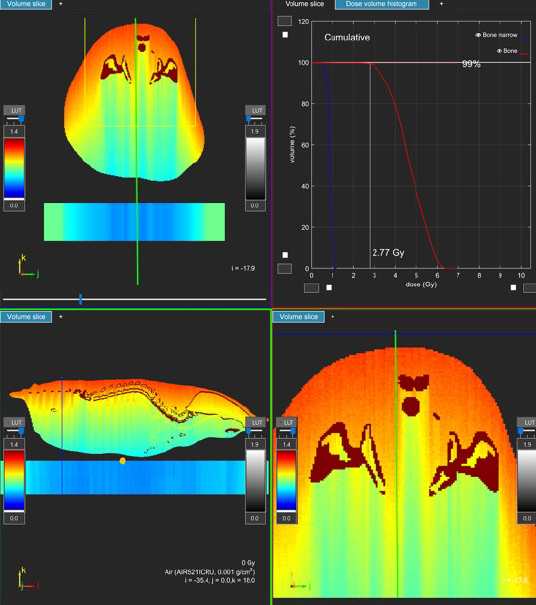
Figure 1: Dose gradient of a 160kVp beam illustrating the absorbed dose through a mouse lying prone.
The dose to the skin on the surface closest to the tube is significantly higher than throughout the rest of the mouse.
Image provided by SmART Scientific Solutions
Summary
Overall, TBI in mice at 225-350 kVp is generally more effective and reliable than 160 kVp. The higher energy provides better penetration, improved dose uniformity, reduced skin toxicity, and more reproducible biological responses, making it the preferred choice for whole-body irradiation studies in mice.
References
Peter, R., Caravaca, J., Yang, J., Gunther, C., Serrano, J. A. C., Nostrand, C., … & Seo, Y. Dose mapping a gamma-ray irradiator and x-ray irradiator to obtain rodent absorbed depth dose equivalence between technologies.
Stasko, J. T., Hammer, C. G., & Culberson, W. S. (2022). The Effect of Mouse Size on Dose from an X-Rad320 Irradiator. Radiation Research, 197(6), 650-654.
